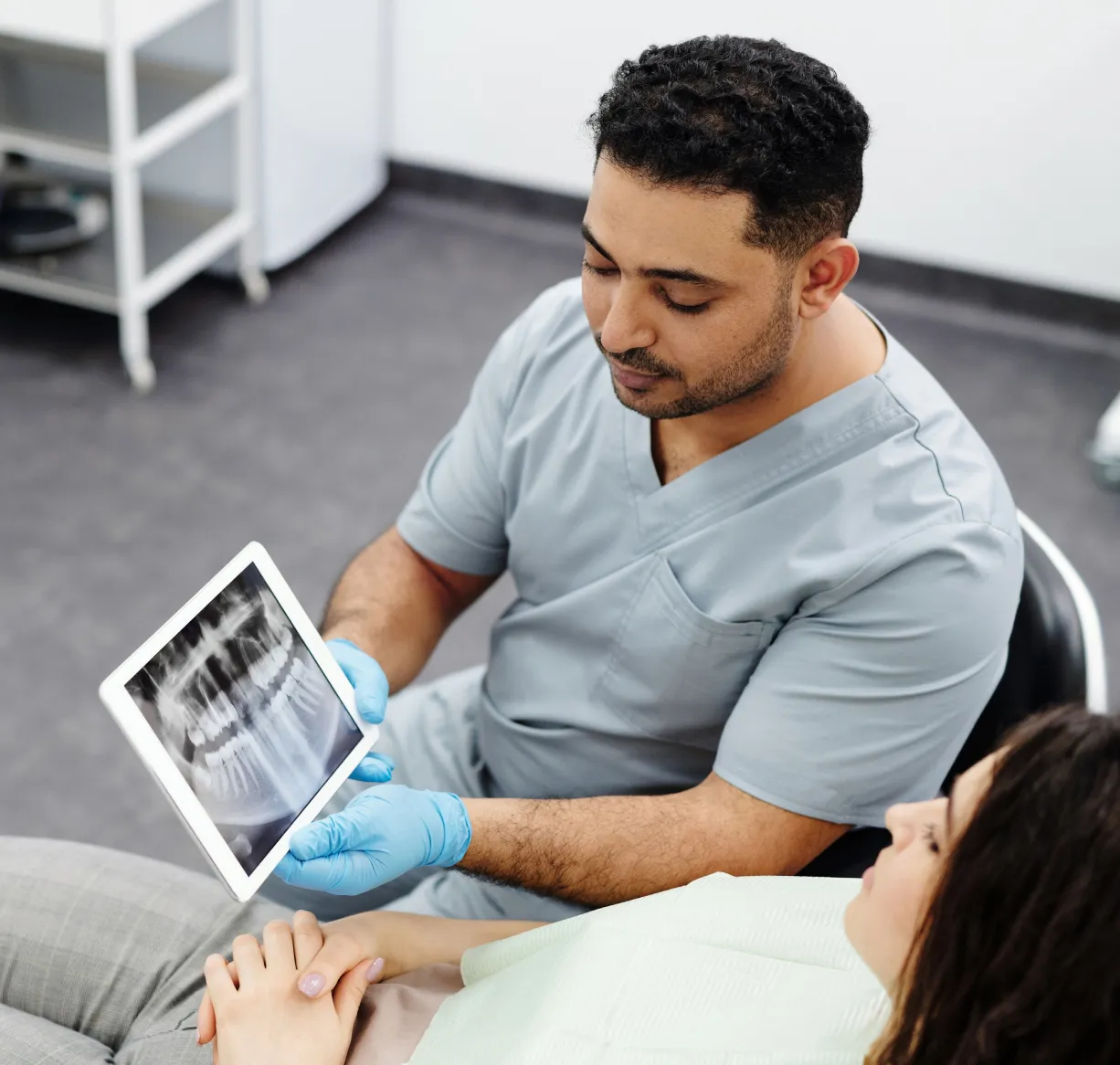
Patient education
Dental implants, all you need to know
Dental implants offer superior durability, natural appearance, improved oral function, and enhanced self-confidence, providing a long-lasting solution for missing teeth.

Product Description
OsseoFuse Dental Implants are manufactured from biocompatible titanium and titanium alloy, and abutments from titanium, titanium alloy, gold alloy and ceramic material. These implants arc designed for surgical implantation into the upper and/or lower jawbone for the attachment of prosthodontic appliances to replace missing teeth.
Indications for Use
OsseoFuse Dental Implants are intended for surgical placement in the upper and/or lower jaw to provide a means for prosthetic attachment in single tooth restorations and in partially or fully edentulous spans with multiple single teeth utilizing delayed or immediate loading, or as a terminal or intermediary abutment for fixed bridgework, and to retain overdentures.
Contraindications
OsseoFuse Dental Implants should not be placed in patients where the remaining jaw hone is too diminished to provide adequate stability of the implant (minimum 1mm circumferential and 2mm apical). Lack of osseointegration or subsequent implant failure may occur in cases where there is insufficient surrounding bone, poor bone quality, poor oral hygiene, heavy smoking, tobacco abuse, or medical conditions such as blood disorders or uncontrolled diabetes.
Direction for Use
For a detailed explanation of the procedural precautions, refer to the Surgical Manual. During the planning phase, it is important to determine the vertical dimension, the actual space available between the alveolar crest and the opposing dentition, in order to confirm that the available space will accommodate the proposed abutment and the final crown restoration.
This information varies with each patient and abutment: therefore it should he carefully evaluated before placing any dental implant. The final prosthesis should be designed prior to the placement of the dental implant. Utilize continuous irrigation with a cool, sterile irrigating solution to avoid excessive damage to the surrounding tissue and to prevent compromising osseointegration.
This is mandatory during all procedures. Avoid excessive pressure during preparation of the bone site. As the drilling speed varies based on the instrument and the surgical procedure, recommendations for speed can be found in the Surgical Manual. Only sharp instruments of the highest quality should he used for any surgical procedure involving hone. Minimizing trauma to the bone and surrounding tissue enhances the potential for successful osseointegration. In order to eliminate contaminants and other sources of infection, all non—sterile devices should be cleaned and/or sterilized prior to use.
Storage and Handling
Devices should be stored at room temperature. Refer to individual product labels and the Surgical Manual for special storage or handling conditions.
Warnings
Implant surgery and restoration involves complex dental procedures. For safe and effective use of OsseoFuse Dental Implants, proper implant surgery training is strongly recommended prior to implant use. Improper technique and patient selection may result in implant failure and/or excessive loss of supporting alveolar bone. Use of electro—surgical instruments or lasers around metallic fixtures and their abutments is not recommended due to the risk of electric shock and/or burns. Excessive mobility, bone loss, or infection may indicate the implant is failing.Any implant that appears to be failing should be treated or removed as soon as possible. If removal is necessary, remove any soft tissue from the implant site and allow site to heal as though it were an atraumatic extraction. Mishandling of small components inside the patients mouth carries a risk of aspiration and/or swallowing.
Precautions
For safe and effective use of OsseoFuse Dental Implants, abutments and other surgical and restorative dental accessories, these products or devices should only be used by trained professionals. The surgical and restorative techniques required to properly utilize these devices are highly specialized and complex procedures. Improper technique can lead to implant failure, loss of supporting bone, restoration fracture, screw loosening and aspiration.
Sterility
The fixture, fixture mount and cover screw have been cleaned and sterilized by gamma irradiation and are ready for use. Do not use sterile products if the packaging has been damaged or previously opened. Do not re—sterilize and autoclave except where instructions to do so are provided on the product label, in the Surgical Manual, in the Restorative Manual or in any additional marketing literature for that product.
Procedural Precautions, Restoration
The healing period varies depending on the quality of the bone at the implantation site, the tissue response to the implanted device and the surgeon’s evaluation of the patient’s hone density at the time of the surgical procedure. Excessive force applied to the dental implant should be avoided during the healing period. Proper occlusion should be evaluated on the implant restoration to avoid excessive force.
Potential Adverse Events
Potential adverse events associated with the use of dental implants may include:
- Failure to osseo—integrate
- Loss of osseo—integration
- Dehiscence requiring bone grafting
- Perforation of the maxillary sinus, inferior border, lingual plate, labial plate, inferior alveolar canal, gingiva
- Infection as reported by: abscess, fistula, suppuration, inflammation, radiolucency
- Persistent pain, numbness, paresthesia
- Hyperplasia
- Excessive bone loss, which might necessitate surgical intervention
- Implant fracture
- Engineered and Designed by Dynamic Innovations Inc. 5023 North Parkway Calabasas, Calabasas, CA 91302.1 Tel. 888) 446-9995 Fax. 310) 356-3183
- Manufactured by : Kjmeditech Co.,1,td. 959-23, )aechon—dung, I iuk—gu, Gwangju Metropolitan—city, Korea. /Tel. 82-62-972-5476 Fax. 82-62-973-2809
Get in touch
We get a lot of questions. You can drop us an email, call us, or text us. You can email or text us your order as well!.
Suite B, Las Vegas, NV
89146